Ozoral PRO - I Class CE Medical Device. PROFESSIONAL USE ONLY.
Ozoral PRO - I Class CE Medical Device. PROFESSIONAL USE ONLY.
Mucoadhesive hydrogel based on 15% of our Ozonized Sunflower Seed Oil named Ozonia 3000.
EAN code 8052787730153
Medical device Class I for the ORAL CAVITY
CND: V9099
pH about 6.4.
Peroxide index about 450 meqO2/kg.
RESERVED EXCLUSIVELY FOR THE DENTAL OFFICE.
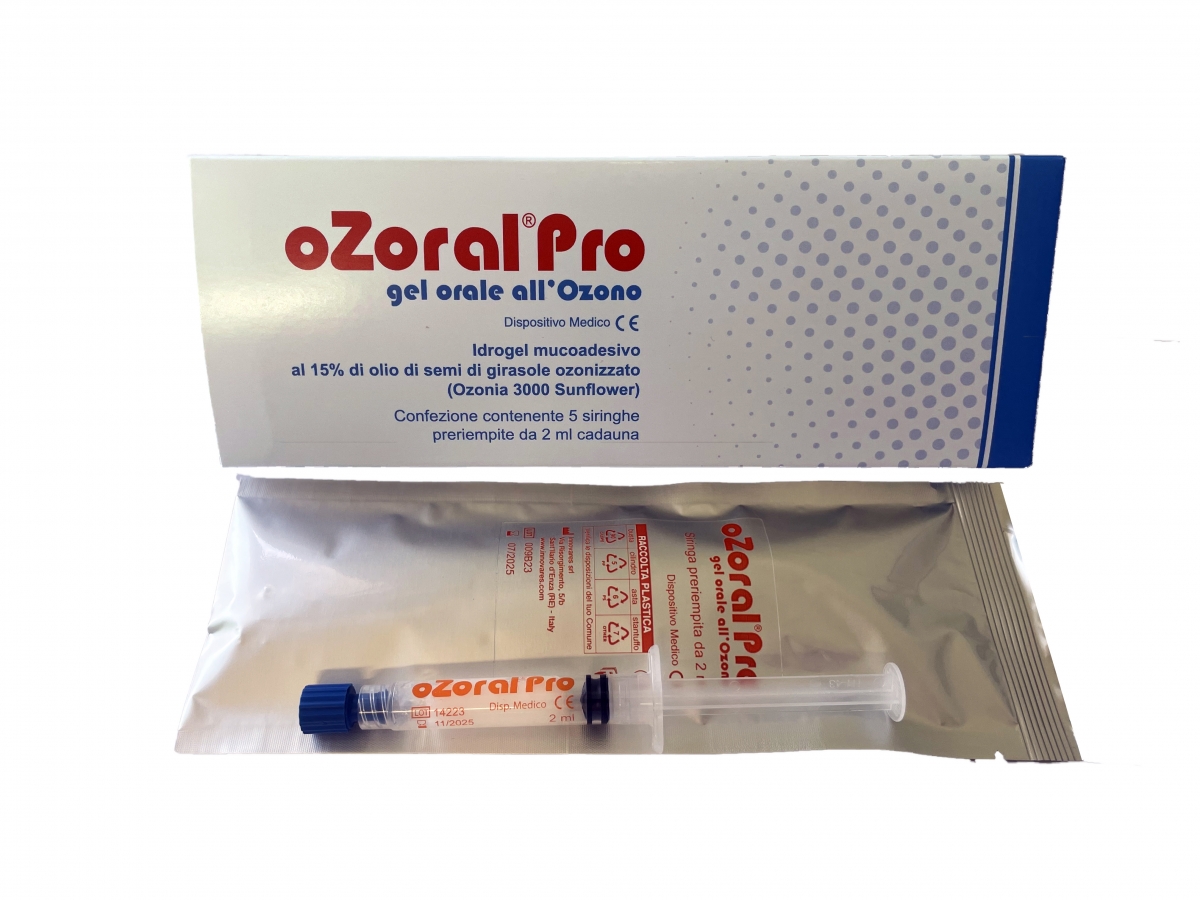
Mucoadhesive oral hydrogel containing 15% of ozonized sunflower seed oil, named Ozonia 3000 Sunflower.
Ozonia 3000 Sunflower is manufactured, titrated (test method ISCO3), standardised and stabilised by Innovares.
Protective (Microbiological Study), soothing, enhancing tissue repair processes.
It supports a healthy oral environment, with particular emphasis on gingival and periodontal pockets and cavities. Ozoral is suitable for the treatment of eroded, damaged or inflamed areas, outcomes of oral surgery and iatrogenic damages in general (thrush, periimplantitis, periodontitis, gingivitis, stomatitis caused by prostheses, lesions caused by orthodontic braces; insult due to tartar removal, root scaling, root planing, etc.).
FUNCTIONAL INGREDIENT
Ozonia 3000 ozonized sunflower seed oil manufactured, titrated, standardized and stabilized by Innovares.
DOSAGE AND INSTRUCTIONS FOR USE
Apply the product after an appropriate oral hygiene regimen. Avoid eating, drinking or rinsing the oral cavity for at least half an hour after treatment.
PACKAGING
Package containing 5 prefilled syringes 2 ml each.
STORAGE
Keep the product at a temperature not higher than 25°C. Do not expose the product to sunlight and sources of heat.
PRODUCT WARRANTIES, QUALITY REQUIREMENTS, AND CHARACTERISTICS
Ozoral Pro has proven to be a non-irritating product.
Ozone is a gas with a pungent odor that has been mitigated in the product: it is, therefore, a sign of the active ingredient’s authenticity and efficacy and it is normally well tolerated.
Want more information about the product
Ozoral PRO - I Class CE Medical Device. PROFESSIONAL USE ONLY.Fill out the form below, you will be contacted by our representative in the shortest time possible
Sending, please wait